Just for the fun of it:
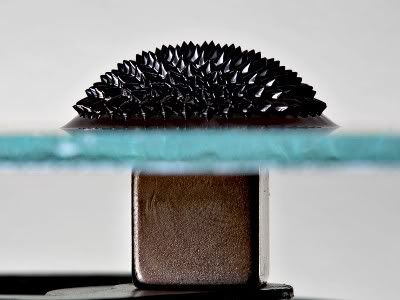
A liquid magnet or ferrofluid is a colloidal mixture of magnetic particles (~10 nm in diameter) in a liquid carrier. The carrier contains a surfactant to prevent the particles from sticking together. Ferrofluids can be suspended in water or in an organic fluid. A typical ferrofluid is about 5% magnetic solids, 10% surfactant, and 85% carrier, by volume. One type of ferrofluid you can make uses magnetite for the magnetic particles, oleic acid as the surfactant, and kerosene as the carrier fluid to suspend the particles.
Several people have asked me if they can make substitutions for the oleic acid and the kerosene. The answer is yes, though changing the chemicals will change the characteristics of the ferrofluid somewhat. You can try other surfactants and other organic solvents. The surfactant must be soluble in the solvent.
When no external magnetic field is present the fluid is not magnetic and the orientation of the magnetite particles is random. However, when an external magnetic field is applied, the magnetic moments of the particles align with the magnetic field lines. When the magnetic field is removed, the particles return to random alignment. These properties can be used to make a liquid that changes its density depending on the strength of the magnetic field and that can form fantastic shapes.
You can find ferrofluids in high-end speakers and in the laser heads of some CD and DVD players. They are used in low friction seals for rotating shaft motors and computer disk drive seals. You could open a computer disk drive or a speaker to get to the liquid magnet, but it's pretty easy (and fun) to make your own ferrofluid.
The full howto is on chemistry.about.com and can be found
here.
edit: Here's a
cool YouTube video demonstrating the way this stuff reacts to a magnetic field.